IRB Process
The IRB process aims to ensure the appropriate use of human participants. The general procedures for submission, review and approval of documents submitted to the IRB are listed below.
If you have any questions, please contact IRB Administration at IRB@uwosh.edu or call (920) 424-3215.
Principal Investigator Eligibility Status
The eligibility status for Principal Investigator (PI) for all research activities at UW Oshkosh has been updated to be congruent with Principal Investigator status for related grant work. Please review SOP #6: Principal Investigator Eligibility.
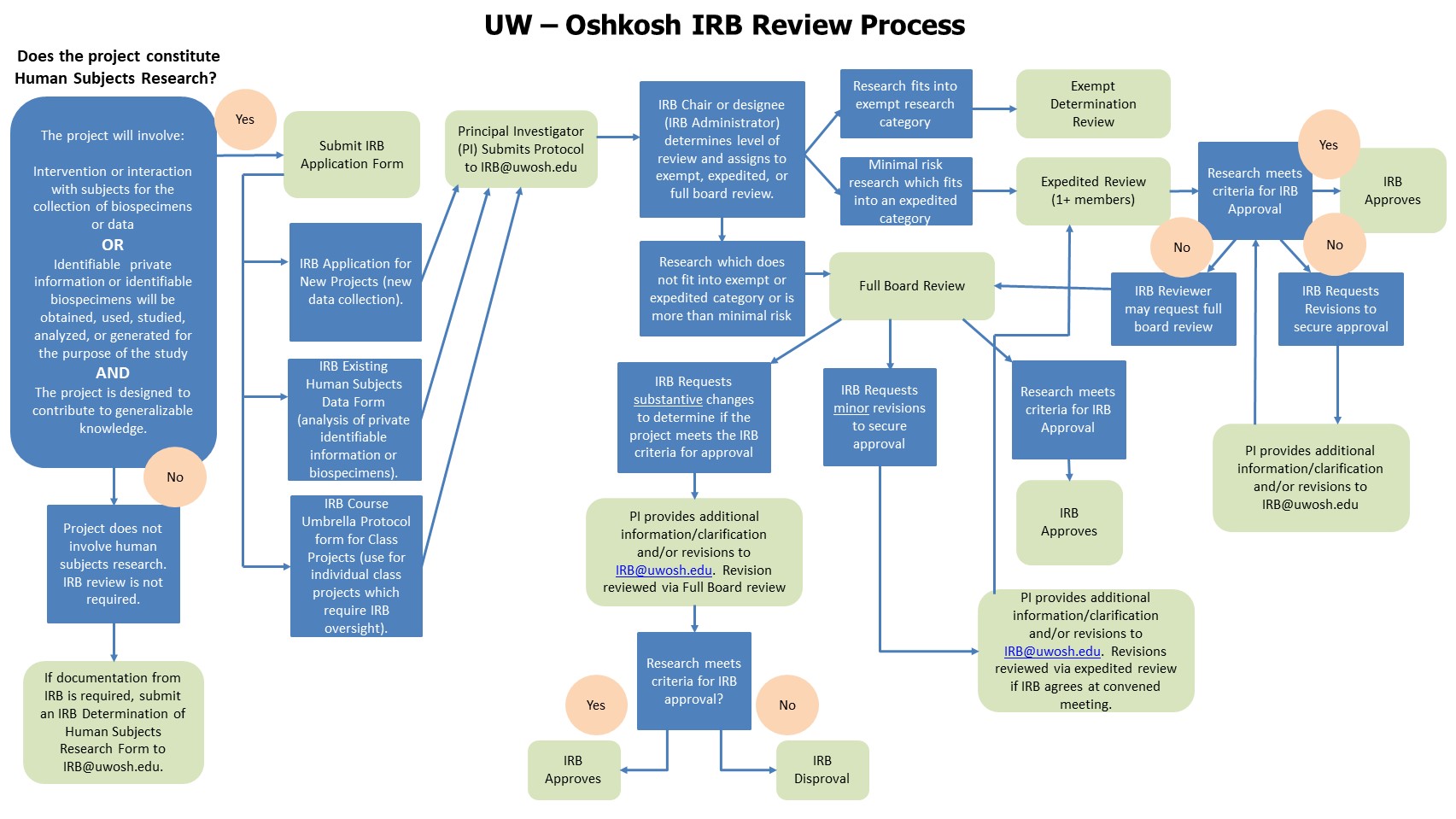
Submission Process
- Determine which type of application you need: (1) IRB Protocol Application for New Projects (for collecting new data),
(2) Existing Human Subjects Data Form (for analysis of an existing data set), or (3) Course Umbrella Protocol Application for Class Projects (only needed when class projects require IRB review). To determine if your class project requires IRB review, see SOP #7: Student Class Assignments. - Submit a signed electronic copy with all appendices as a single word or pdf document to IRB@uwosh.edu.
- Do not begin recruiting participants or collecting data until IRB review is complete and you have received confirmation of approval.
- To determine if an IRB Application submission for formal review is required for quality improvement, quality assessment or program evaluation activities, please submit the following form: IRB Determination of Human Subjects Research Form for Quality Improvement/Quality Assessment Activities.
- All forms are located on the IRB Forms page.
Review and Approval Process
- Determine which type of application you need: (1) IRB Protocol Application for New Projects (for collecting new data),
(2) Existing Human Subjects Data Form (for analysis of an existing data set), or (3) Course Umbrella Protocol Application for Class Projects (only needed when class projects require IRB review). To determine if your class project requires IRB review, see SOP #7: Student Class Assignments. - Submit a signed electronic copy with all appendices as a single word or pdf document to IRB@uwosh.edu.
- Do not begin recruiting participants or collecting data until IRB review is complete and you have received confirmation of approval.
- To determine if an IRB Application submission for formal review is required for quality improvement, quality assessment or program evaluation activities, please submit the following form: IRB Determination of Human Subjects Research Form for Quality Improvement/Quality Assessment Activities.
- All forms are located on the IRB Forms page.
Continuing Review
- To determine if formal Continuing Review is required for your project, please review your most recent IRB Approval Memo and note the protocol expiration date if applicable.
- If continuing review is required, you will need to submit an IRB Continuing Review Form, with a brief summary of the project.
- If you wish to continue your research, you must request an extension. An extension request must be approved by the IRB before continuing the study beyond the project approval period. The IRB requests that continuing review forms be submitted at minimum 14 days prior to protocol expiration. Submitting an extension request in sufficient time is the responsibility of the PI.
- If your protocol expires, you must stop all human subjects research related to that project. You have 30 days to submit a continuing review form in order to keep that study open. Studies not renewed within that 30 day window will be closed administratively and a new application will need to be submitted to the IRB for review in order to continue the human subjects research.
- If you have completed your research at the time of continuing review, please submit an IRB Closure Form.
- If continuing review is not required for your project, the IRB will send an annual check-in email.
- If you have completed your research, please submit an IRB Closure Form.
Modifications
If changes to your research project are requested, you must submit an IRB Modification Request Form. Approval for your modification request must be granted by the IRB before the changes to your protocol can be implemented.
Closures
Researchers (PIs) must close out their IRB protocol once the research project is complete. The IRB office will send out reminder e-mails to PIs at 60 and 30 days prior to protocol expiration. For protocols that do not have an expiration date assigned, the IRB will send an annual email to check on the status of the protocol. Protocols can be closed by the PI by submitting the IRB Closure Form to IRB@uwosh.edu.
Adverse Event and Problem Reporting
Any adverse event or unanticipated problems must be reported to the IRB within 72 hours of the event using the Unanticipated Problem Form or an Adverse Event Form.
Hiring a volunteer
- If you wish to have a non-affiliated person (not associated with an institution of higher education) collaborate on a UW Oshkosh led project involving human subjects research, please contact the HR Office and notify them that you wish to hire a volunteer.
- Download and complete the two required forms below:
- Return these documents to the HR Office.
- HR will follow up with all parties (supervisor, volunteer, etc.) involved and will determine if a criminal background check is necessary.
If the volunteer needs driver authorization, contact the Parking Office at 920-424-4455, visit: https://www.uwosh.edu/riskmanagement/driver-authorization-management
Related Policies
Contact IRB
IRB Chair
Anca Miron
mirona@uwosh.edu
IRB Administrator
Kelly Schill
(920) 424-3375
IRB@uwosh.edu
Office located in Dempsey Hall Suite 214
OFFICE OF SPONSORED PROGRAMS
Our Team Members
Office Contact
Phone: (920) 424-3215
Email: OSP@uwosh.edu
Office Location: Dempsey 214
Mailing Address: 800 Algoma Blvd. Oshkosh, WI 54901-8627
Our Team Members
Office Contact
Phone: (920) 424-3215
Email: OSP@uwosh.edu
Office Location: Dempsey 214
Mailing Address: 800 Algoma Blvd. Oshkosh, WI 54901-8627
